Wet-chemical methods approaching magnetocalorics: Rapid sol-gel synthesis of the magnetocaloric antiperovskite phase Mn3GaC
New publication
2023/11/06
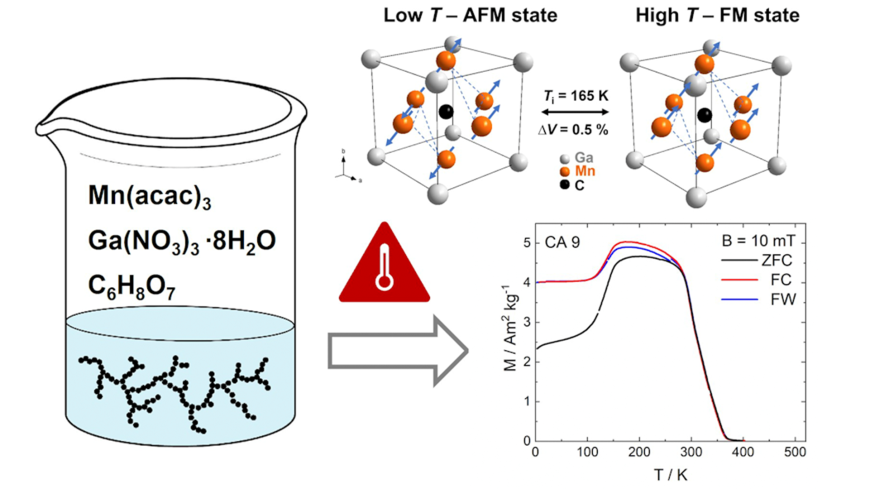
One promising member within the group of magnetocaloric materials is the antiperovskite phase Mn3GaC. It shows a first-order transition from a low-temperature antiferromagnetic state to a high-temperature ferromagnetic state at around Ti = 165 K. The inverse magnetocaloric effect combines an adiabatic temperature change of ∆Tad of -5.4 K in a field change of 2 T (sample cools upon applying a field), combined with a large entropy change (∆ST = 15 J kg-1 K-1) and a narrow thermal hysteresis of 4 K. However, despite the big potential that lies in its materials properties, the synthesis of this phase, and antiperovskites in general, is still mainly achieved by conventional energy consuming solid-state methods, resulting in long reaction times (>7 days) and challenges in influencing the microstructure or morphology. Here, we report a rapid sol-gel approach using citric acid as a gelling agent, which allows us to obtain a highly phase pure, crystalline Mn3GaC phase after only 5 hours of annealing. Ex situ X-ray powder diffraction data, combined with DSC/TGA measurements elucidate the reaction mechanism which involves carbothermal reduction of intermediate oxides. Vibrating sample magnetometry of the annealed products demonstrate the ability to alternate the magnetic properties of Mn3GaC simply based on the variation of the gelling agent amount in the reaction mixture. Overall, this offers a facile alternative, energy- and time efficient approach for the synthesis of antiperovskite carbide phases with the power to specifically influence materials properties by adjusting only small parameters within this wet chemical-based synthesis procedure.
N. Kubitza, P. Babaei, U. Wiedwald, C. S. Birkel
Chem. Mater., 35, 21, 9175–9181 (2023).



