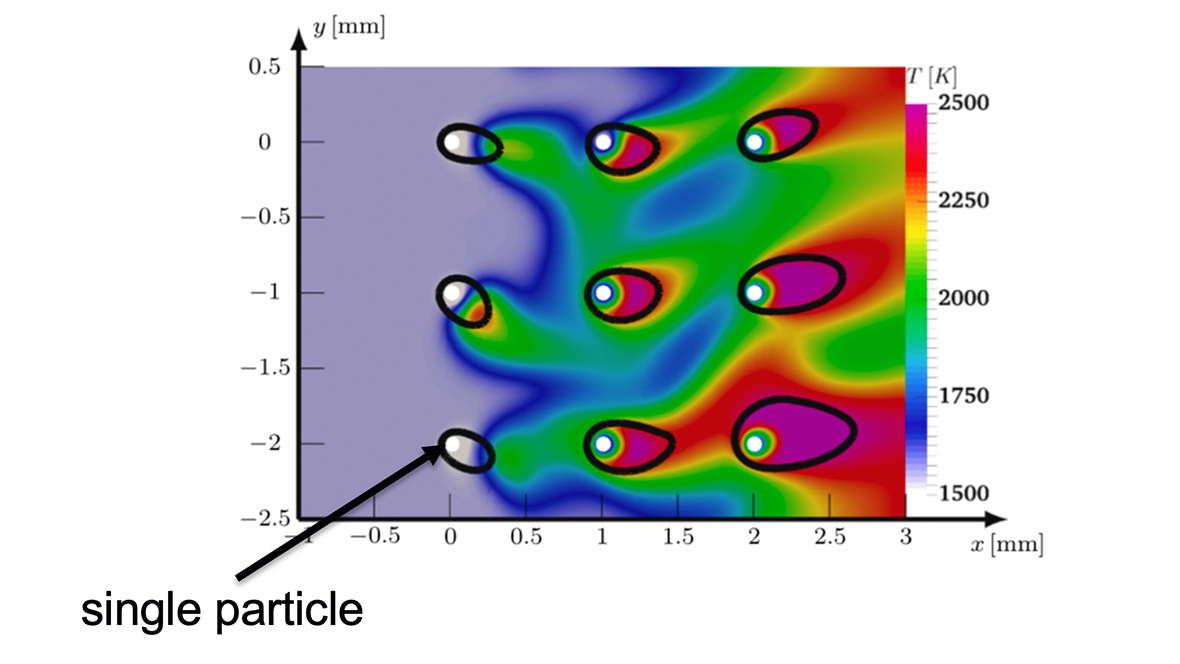
In thermo-chemical reduction, porous microparticles of iron oxides are reduced with green hydrogen to porous microparticles of pure iron. The thermo-chemical oxidation of the iron particles with atmospheric oxygen to iron oxide particles represents the complementary process and closes the cycle. For reduction and oxidation, the reactions and their coupling to transport processes must first be understood for individual particles, since both heat and mass transfer between particles and the surrounding gas phase and the kinetics of the heterogeneous surface reaction can potentially be rate-determining processes. Both are strongly influenced by the ambient conditions (temperature, composition of the gas atmosphere) and the particle properties (particle size, porosity, etc.).
In this subproject, a detailed single particle model for the thermo-chemical conversion (reduction and oxidation) of micro-sized particles will be developed in order to identify and model rate-controlling processes depending on the above-mentioned influencing factors. For this purpose, the experimental results, especially the heterogeneous surface kinetics, have to be integrated into the particle model. The development of the particle structure (degree of oxidation, porosity development) is basically opposite for reduction and oxidation, but can be described mathematically almost analogously. Since the particles undergo both processes alternately and repeatedly, the final state of one process is the initial state of the other.
Scientific questions:
- How do the total reduction or total degree of oxidation and the distribution of oxidation states in the particle behave as a function of time?
- What residence time is required to completely reduce iron oxide particles to iron particles in different hydrogen-rich reducing atmospheres?
- Which kinetic parameters influence ignition delay time and burning rate, i.e., the time for full oxidation, during thermo-chemical oxidation?
- What is the uncertainty of ignition delay time and burning rate due to limits in measurement precision?