Clean Circles

Reduction Processes
Renewable Energy Storage by Reduction of Iron Oxides
Research Area 1 focuses on the reduction of iron oxide in order to store electric energy obtained from renewable sources. Reduction is investigated in two different approaches: electrochemical and thermochemical reduction.

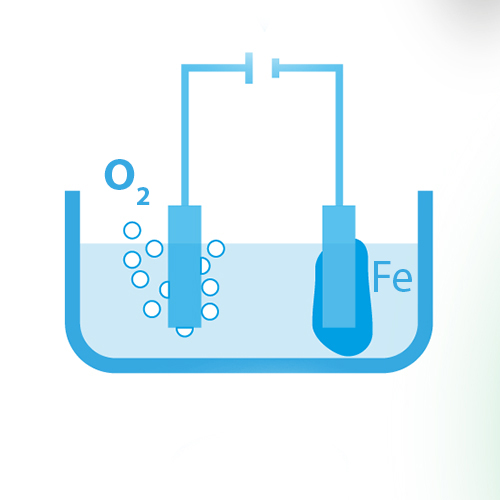
Electrochemical reduction
The electrochemical reduction of iron oxides dissolved in ionic liquids is investigated regarding the reaction kinetics and thermodynamics in half-cells. Phase transitions during dissolving iron and its reductive separation are studied in full cells and a Taylor-Couette test bench. Results are used to develop reactor concepts.
Thermochemical reduction
The sequence of investigations of thermal reduction processes ranges from heterogeneous reaction kinetics to flow reactors, in which chemical reactions couple with physical transport processes, to fluidized beds.
Milestones
- Define process conditions and range of parameter values
- Setup of a rough kinetic and thermodynamic database from parameter studies
- Setup of a detailed kinetic and thermodynamic database from parameter studies
- Analyze coupled transport and thermodynamic reduction processes
- Complete all steps along the process chain for the thermochemical and electrochemical reduction
Research area coordination
| Name | Contact | |
|---|---|---|

| Prof. Dr. Ulrike Kramm | ulrike.kramm@tu-... +49 6151 16-20356 |

| Prof. Dr. Olaf Deutschmann | deutschmann@kit.edu +49 721 608-43064 |
Projects in research area 1
Experimental investigation of particle clouds
Kinetic model
Fluidized bed
Thermo-chemical reduction/oxidation in fluidized beds (B. Epple)
Dissolution and electrochemistry
Laser-induced plasma spectroscopy (LIBS)
Hard X-ray studies
Dissolution and motion dynamics
Dissolution and motion dynamics of metallic microparticles in ionic shear flows (J. Hussong)
Mössbauer spectroscopy
Structure-property relationships (X-Ray Scattering)
Structure-property relationships of iron particles and its oxides (H. Nirschl)



